Our second feature in this mini-series on the human microbiome explores skin health, which is far more complex as well as exciting in terms of addressing important skin health opportunities. In this feature Elric Langton looks at two very different British Companies, both leading the world in skin care products, and science.
You can read the first part of this feature here.
Croda International Plc (LON: CRDA)
Croda International Plc has divested most of its Performance Technologies and Industrial Chemicals businesses recently to a wholly owned subsidiary of Cargill Inc, in a strategic move that positions Croda firmly in consumer care, with a strong focus on the skin healthcare markets, including cosmetics through wholly owned subsidiary Sederma. The divestment means Croda has a war chest of c£667 million, which has a few investors a little nervous about their investment in another company at the other end of the market value chain. More on this later.
Croda remains an ingredients Company but a more focused Company that uses smart science to create high-performance ingredients and technologies that aim to improve consumer wellbeing, beauty, care, fragrances and improve lives using emerging microbiome technology, not only with its own science and products, but also by developing partnerships with Companies which complement Croda’s new strategy.
Steve Foots, Chief Executive of Croda, said: “This divestment accelerates Croda’s transition to being a pure-play Consumer Care and Life Sciences company. We will redeploy capital and resources to scale our consumer, health, and crop care technologies, helping to deliver consistent, superior sales growth and even stronger profit margins.”
Croda is not afraid to wield the checkbook if it is to further advance its market penetration. In 2013 Croda International acquired a 65% equity interest in Sichuan Sipo, a natural specialty chemicals manufacturer based in China. In 1995 Croda acquired Sederma, a French chemical Company for $65 million. This was a key event which has seen Croda dominate the cosmetics industry through Matrixl, the world’s most popular peptide used in cosmetics to reduce the appearance of fine lines.
The microbiome, which is known to be linked with the inflammatory process, its activation and control pathways, offers a potential route supporting the natural immune-inflammatory response within products such as sunscreens. In February 2019, the US FDA issued guidance to remove two common ingredients from cosmetics and sunscreens (aminobenzoic acid and trolamine salicylate) and is in the process of evaluating further safety data on 12 other widely used ingredients, with many of the chemicals detected on the skin and in the blood weeks after application. This has created a need within the sunscreen and cosmetics industries for more naturally derived ingredients to support the body’s immune-inflammatory response to different environmental challenges.
This is where my next Company fits in.
SkinBioTherapeutics (AIM: SBTX)
SkinBioTherapeutics was divested out of OptiBiotix Health Plc (AIM: OPTI) in April 2017 at 9p, unlocking seemingly hidden value from OptiBiotix. By doing so it reduced a cash drain on OptiBiotix, more importantly, it allowed SkinBioTherapeutics to develop its science and IP at its own pace. Prior to this it was known as SkinBiotix, a dermatology focused project born at the University of Manchester (UOM). In March 2016, a technology created from basic science research within UoM laboratories was spun out into a commercial vehicle called SkinBiotix ltd. In April 2017, SkinBiotix Ltd was floated on the AIM market of the London stock exchange and became SkinBioTherapeutics plc.
The Company was put under the stewardship of Pro Catherine O’Neil, who became the first stand-in CEO while the Company developed its IP. The Company soon attracted a CEO with the right credentials to move the Company up a gear or two. The current CEO is Stuart Ashman, a charismatic Newcastle United fan, with incredible drive, and very interesting contacts from his previous roles I believe will prove rewarding for the future. Indeed, Stuart has performed remarkably well during his tenure given he has had to deal with Covid-19, which impacted on the Company’s original plan to put the AxisBiotix Ps probiotic through clinical paces. If successful would have allowed for specific health claims in marketing literature. However, as we know, all available lab space was utilised to fight Covid-19, which meant Stuart and his small team had to change its strategy for the probiotic, which involved a very successful human study. More on this later.
SkinBioTherapeutics (SBTX), a life science company focused on skin health. It has five technology platforms, where at the moment focus is on low hanging fruit – SkinBiotix and AxiBiotix, and soon, MediBiotix. Both AxisBiotix and SkinBiotix already have strong commercial partnerships already generating recurring revenues. AxisBiotix has proved to be a successful part of the first pillar to produce a commercial consumer facing probiotic designed to alleviate symptoms of psoriasis. SkinBiotix via its science validated lysate covering a broad cosmetic intel inside active ingredient. The term “Cosmetic” is a catch-all term and includes not only what we think of as cosmetics i.e., face creams, but also sun, bath and shower, hands, scalp and hair, deodorants etc.
Overview
The Company had initiated a research program with the University of Manchester to investigate and develop microbiome formulations that support natural anti-inflammatory response to a range of environmental challenges. Expanding the university’s collaboration was one of the areas of investment laid out by the Company when it raised funds via placing an open offer in October 2020. This is what we now know as “low hanging fruit” allowing SkinBioTherapeutics to develop consumer-facing products early, therefore generating revenues preserving existing cash, limiting shareholder dilution.
AxisBiotix Bright consumer study for psoriasis and extending the scope of the study to enable the inclusion of an additional cohort of participants with non-psoriatic conditions, e.g. Acne, Eczema, and Rosacea.
Important patent grant received for SkinBiotix in medical, cosmetic, and wound care indications in the US and Japan

The first commercial pillar is SkinBiotix
An agreement with FTSE100 stalwart, Croda International for the commercial development for the SkinBiotix lysate. So, what about the lysate?
The SkinBiotix lysate has passed all major obstacle across development risks (manufacturing scale-up, clinical safety/tolerance) – all these have been overcome. Additionally, efficacy studies assessing effectiveness, dose demonstrated no safety issues at high dose there is always potential to up the dose to enhance efficacy if needed.
It is crucial to note that any new cosmetic containing the SkinBiotix lysate allows for a significant advantage to all other cosmetics that does not contain the SkinBiotix lysate. For the first time, allowing for specific claims in marketing material for product “X” can alleviate/reduce fine-line wrinkles, in other words, anti-aging claims – not smoke and mirrors marketing. This is potentially huge for the cosmetic industry, especially Sederma’s clients.
Highlights
- Scientific validation
- Commercial validation
- Access to 12000 + Croda clients
- Access to some of the biggest names in cosmetics
- Option to buy lysate at a reduced cost
- C£5 million investment in a lysate dedicated production line
Cosmetic Skincare
Sederma, the division of Croda Plc that specialises in the manufacture of bioactive ingredients for the cosmetic industry, has updated the Company on the progress of its ongoing collaboration and confirmed that.
- Sederma has successfully replicated the lysate manufacturing process and achieved the same performance from the SkinBiotix technology as established by the Company.
- Sederma has successfully validated scale-up of the manufacturing process at different volume levels.
- During scale-up additional skincare benefits have been identified providing further commercial scope yet to be announced.
- The project is progressing in line with the original plan and has not been adversely impacted by COVID-19.
Sederma will be responsible for the development, manufacture, and commercialisation of the SkinBiotix technology, with any products resulting from this program being sold to Croda’s global portfolio of Personal Care customers. SkinBioTherapeutics will be paid tiered royalties based on global sales revenues on any licensed products derived from the partnership.
The second pillar, AxisBiotix is already producing revenues 12-18 months ahead of schedule. The commercial partnership with Dutch outfit, Winclove Probiotic BV saw them tasked with formulating the AxisBiotix probiotic. This is a considerably difficult task which involves marrying different bacterial strains while their duty is to fend each other off. Nevertheless, Winclove managed the task much sooner than anyone anticipated. For example, sane investors were expecting a commercially viable probiotic on the shelves by 2023. AxisBiotix was launched on 29 October 2021.
There is strong scientific evidence pointing to a link between gut dysfunction, stress-induced alterations to the gut microbiome and skin inflammation. The partnership with Winclove, which was initiated by our CSO, Prof. Cath O’Neill, create a specific probiotic food supplement that has the potential to help manage the hard-to-treat symptoms of psoriasis. SkinBioTherapeutics’ strategy to develop new avenues of microbiome-based technology, this time focused on the gut-skin axis. It also represents the second phase of our strategic plan, following the agreement with Croda plc in the active skincare sector.
The program focusses on how the microbiome can influence and rebalance the body’s response to inflammation in skin health and skin disease. As the body’s biological defense system, the immune system can recognise potentially damaging agents, remove them, and trigger repair. Inflammation is a key component of this immune response. However, the immune response can sometimes become overstimulated, leading to chronic inflammation, which is characteristic of some skin diseases, e.g., atopic dermatitis, acne, and rosacea. Prolonged inflammation is also associated with chronic, non-healing wounds. Winclove is working on the next probiotic formula to target acne.
Working with the translational dermatology team at Manchester University, SkinBioTherapeutics has already identified several inflammatory pathways of interest and routes to target these pathways with microbiome-derived regulators. Subject to a positive program outcome, the Company intends to pursue commercialisation of any identified bacterial formulations through their addition to existing third-party products or the development of new products targeted at specific conditions.
Stuart Ashman, CEO of SkinBioTherapeutics, said:
“This research program with the University of Manchester aims to widen our understanding of the role that the microbiome plays within the immune system and how it can be used to support the body’s natural immune response. Similarly, to our approach with the AxisBiotix food supplement, we hope to eventually bring immune-supporting microbiome formulations to market through everyday products, such as skin lotions and creams, where we see an increasing consumer preference for natural ingredients. This is an exciting new area of research for us and has the potential to broaden our skin health pipeline further.”
Prof Cath O’Neill, CSO of SkinBioTherapeutics, said:
“There is now an extensive growing body of research demonstrating direct links between the microbiome and the immune system, and consequently skin health. The Company is in the middle of a food supplement study and the potential modifying effect on skin conditions, such as psoriasis. However, with this new line of research, we have the opportunity to assess the powerful effect of the microbiome on the immune system and skin health.”
AxisBiotix Ps
Participants suffering from mild to moderate psoriasis will be invited to participate in a human study in which they will be provided with samples of AxisBiotix (TM)Ps to self-administer over an eight-week period and track the impact of the food supplement on their skin condition. Participants will submit their findings periodically through a bespoke mobile device app, thus avoiding the need for clinical attendance. This will accelerate the timing of readout compared to the previously envisaged conventional study. As a result, if the findings are positive, this will allow for a significantly earlier commercial launch than originally planned. The Company anticipates commencing the trial in early 2021.
It has perhaps taken a little longer to recruit the correct caliber of support staff to really push AxisBiotix commercially, and a little longer to execute marketing strategy. However, there are good signs the additional staff, paid influencers and national adverts are working. Moreover, margins are 65-70%. Of course, retention and subscription rates will fluctuate. Still, with new launches across Europe and ROW, and with the additional commercial distributor awaiting sign-off, there is very real hope, if not expectation, sales will ramp up very quickly.
Bright Study


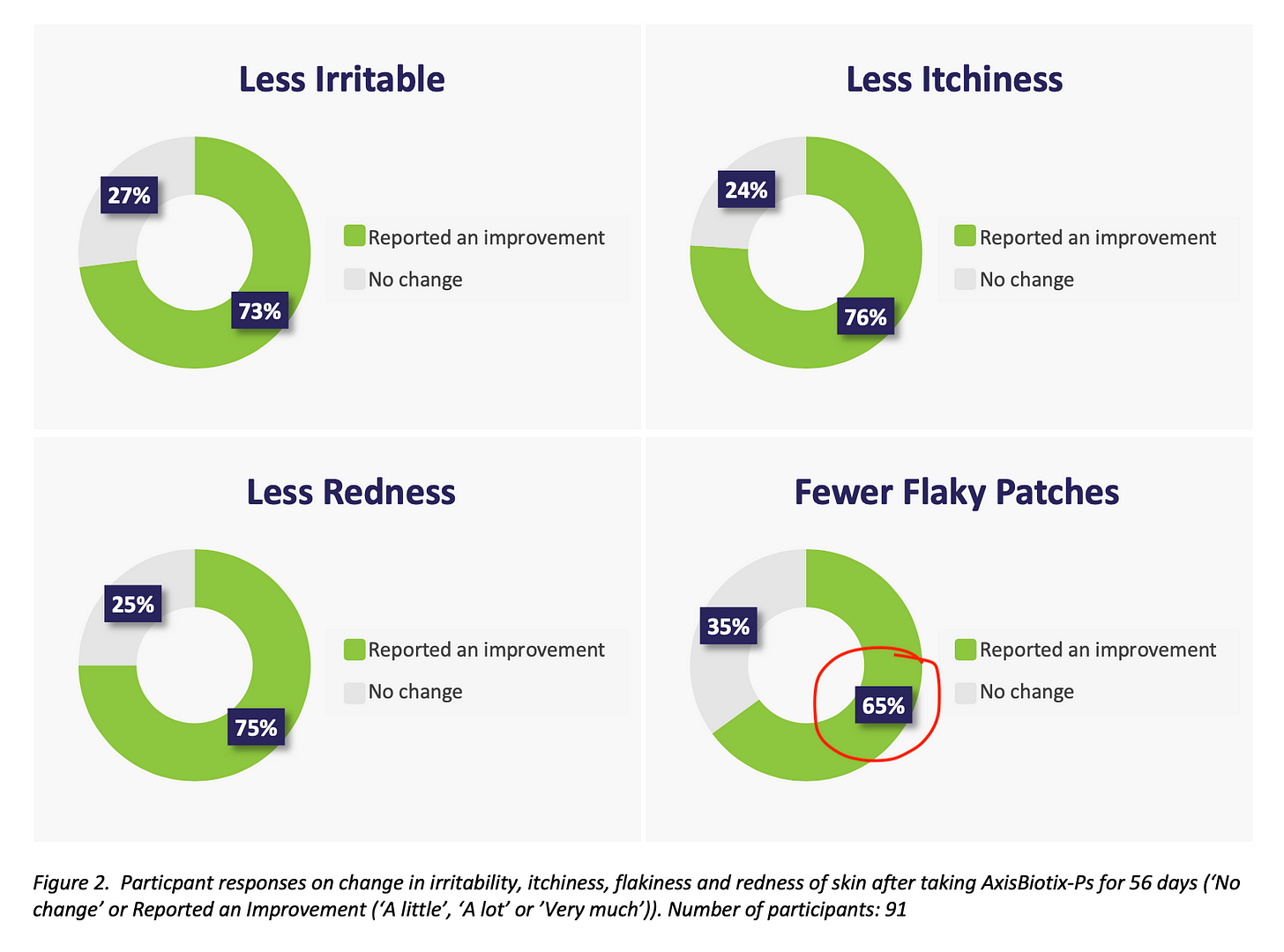
After speaking to a study participant, who had the following observation to share, I would also like to mention. “If the study had been longer than 56 days, the flaky patches reading would probably have also been >70%, as the plaque build-up takes longer to work through than the other symptoms.”
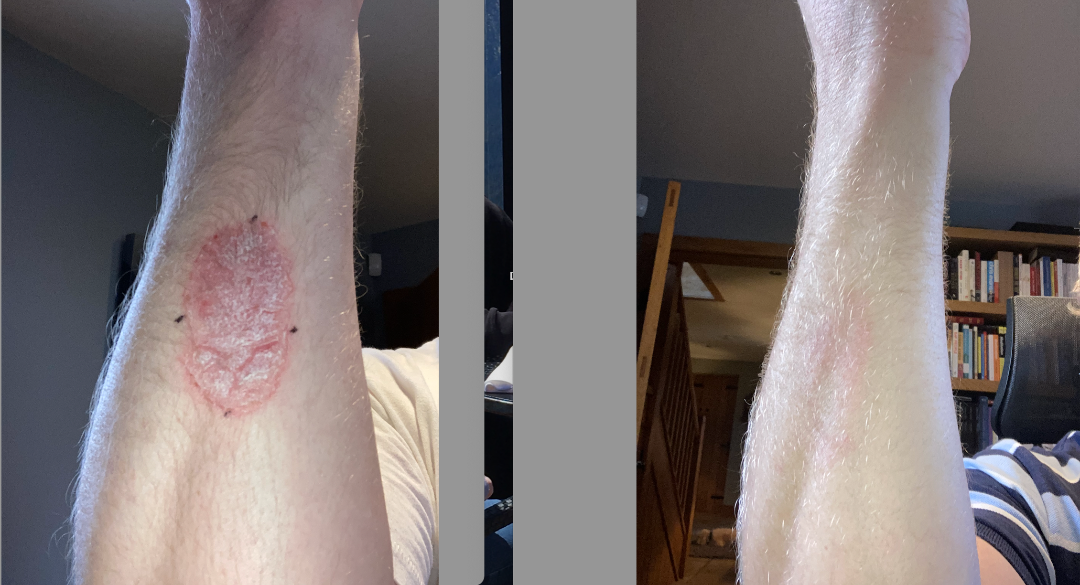
It is only one illustration (below), so we cannot make too much of an emphasis without more consumer feedback, which is gaining momentum. For more illustrations from the Company website.
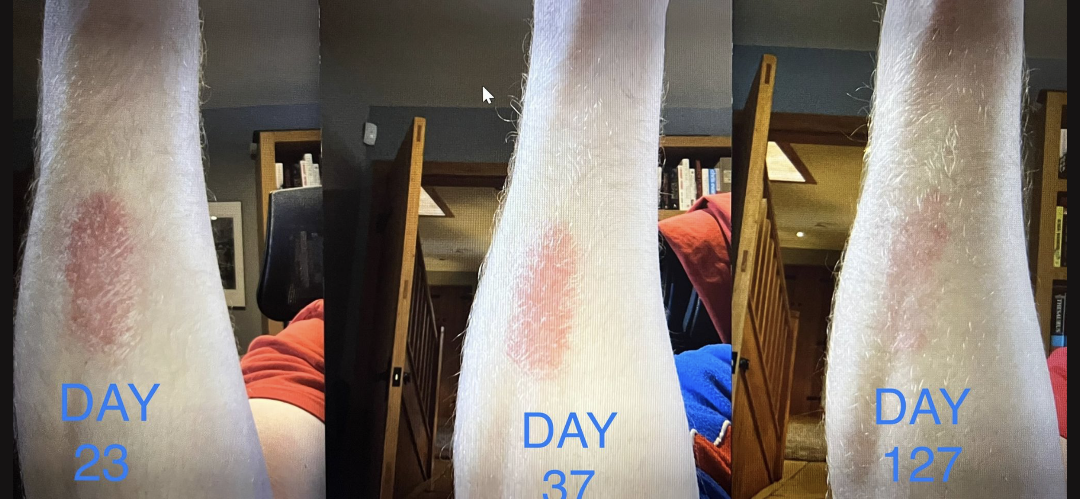
The second example is from the Bright study. On the left is day one. On the right is after eleven months of continued routine with AxisBiotix-Ps.
It is difficult for people who do not have Psoriasis to understand various issues. The flaky skin is the most prominent symptom, but the irritability, itchiness and discomfort can be a circular battle. The graphs below are typical examples of the order of reduced ailment. Flaky skin takes several weeks, if not months before any significant visual improvements are seen. Of course, not all timelines are the same because of the gut flora and lifestyle impact on the probiotic.
Looking Forward
Croda and its active ingredient arm, Sederma, continue to progress and remain on schedule with the development of SkinBiotix as the foundation for an active skincare ingredient for the cosmetic and skin care industry. Indeed, we also learned of additional valuable benefits during scale-up, which, if proven, could add value to the value of the royalties and broaden the market scope. The final scale-up to the capacity required to mass-produce the project is progressing.
Croda has engaged with VIP clients within its portfolio of *12,000+ global cosmetic customers for its inclusion. News is expected ahead of revenues from the partnership, which are anticipated by the year’s end. Let us not forget that other news from the oral care study is expected by the end of this summer. In addition, Sederma will open the lysate door to the remaining *client list. For now, we await to see which of the VIP clients launch a cosmetic with the SkinBiotix lysate as the intel inside.
We also wait for new territories for AxisBiotix, as well as a distribution partnership.
I also expect Winclove to produce another probiotic targeting acne before much longer.
The MediBiotix third pillar is looking very encouraging. I expect significant news shortly, and a third commercial partnership.
Elric Langton of Small Company Champion
Small Company Champion – Substack
This article is for educational purposes only. It is not a recommendation to buy or sell shares or other investments. Do your own research before buying or selling any investment or seek professional financial advice.
This article is for educational purposes only. It is not a recommendation to buy or sell shares or other investments. Do your own research before buying or selling any investment or seek professional financial advice.

